Unique identification of medicinal products
The GS1 system has been used in Switzerland for over 30 years for the identification and labeling of medicinal products. Until today almost 100 percent coverage of Swissmedic registered products has been achieved.
The identification and labeling of medicinal products across all package hierarchy are one of the cornerstones for the digitization of medication processes. They enable applications such as the federal law about the electronic patient record, the electronic vaccination card and the digitization of supply chain and clinical processes.
Identification as added value
The information is displayed by means of a barcode directly on the respective printed on the packaging level and are to all users along the supply chain and the clinical patient pathway available. Through the direct imprint of the identification keys and associated attributes on the packaging, lot of added value is generated. This information can be used at every station of the supply chain and the clinical patient pathway can be recorded directly and transferred to the IT systems.
Unambiguity increases safety
Due to the worldwide uniqueness of the identification keys, unlike proprietary identifications, errors are excluded. At the same time, patient safety is enhanced and supply chain processes made more efficient.
Agreement on uniform standard
In Switzerland, the stakeholders agreed 30 years ago to use the GS1 system and to identify medical products with a Global Trade Item Number (GTIN) In the meantime, most countries worldwide have decided to use the GS1 system for the identification of medicinal products .

Basics of medicinal products and labeling
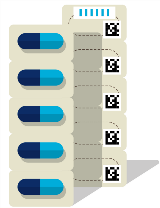
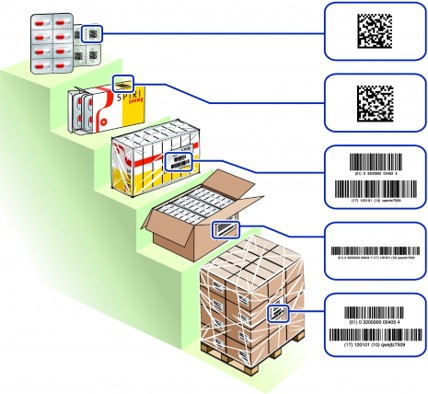
Package hierarchy of medicinal products and their labeling
- Primary packaging
- Secondary packaging
- Tertiary packaging (e.g. bundle packaging)
- Quartenary packaging (e.g. carton and/or pallets)
| Package hierarchy | Product identification |
|
|
|---|---|---|---|
| Primary packaging | GTIN A, like 0 76 12345 00253 x |
GS1 DataMatrix | |
| Secondary packaging | GTIN B, like 0 7680 12345 678 x |
GS1 DataMatrix and/or EAN-13 | |
| Tertiary packaging (multipack/hospital pack) | GTIN C, like 0 76 12345 00254 x |
GS1-128 | |
| Quartenary packaging (carton and/or pallets) |
GTIN D, like 0 76 12345 00255 x |
GS1-128 |
Information on the labeling of primary packaging
Primary packaging is marked with a GS1 DataMatrix, which serves as a minimum information containing GTIN (product identification) and a lot number and ideally also the expiration date. Which information is contained in the GS1 DataMatrix depends on the space available for encryption. If possible, all information should be included in the barcode , but the data content has a direct impact on the barcode size. On the HRI (Human Readable Interpretation or plain writing line) can be waived for primary packaging if this is not possible for reasons of space. The award of the primary packaging is the basis for the digitization,the medication process and the bed side scanning process in the clinical patient pathway.
Information on the labeling of secondary packaging
Secondary packaging is marked with a GS1 DataMatrix, which contains the information GTIN (product identification), lot and expiry date contains. In the future, the GS1 DataMatrix of the secondary packaging will also include the unique serial number, which is used in the execution of the FMD (Falsified Medicine Directive). Besides, under or above the GS1 DataMatrix, there is an HRI (Human Readable Interpretation or plain writing line) to be attached, which the coded values. As there is no guarantee today that all POS systems in pharmacies and drugstores have 2-D Sacanners that are able to process GS1 DataMatrix Barcodes, an EAN-13 barcode can be printed on another packaging. This ensures that the barcode can be captured even with older POS systems. Labeling the packaging with a GS1 DataMatrix allows to add attributes to the barcode (Lot, EXP, Serial-Number) that can be read by the scanner and send them to the downstream IT systems. This enables advanced functions in POS systems, such as the control of the expiry date before of dispensation and the possibility of fully automatic storage with a robot.
Information on the labeling of tertiary packaging
Tertiary packaging is marked with a GS1-128 barcode containing the information GTIN (product identification), lot and expiry date. Next to, below or above the GS1-128 barcode, an HRI (Human Readable Interpretation or plain writing line) is to be placed, which shows the encoded values. For tertiary packages, a one-dimensional symbol is used because it is assumed that such packages could be stored and retrieved by an automated logistics system that cannot necessarily process a two-dimensional symbol.
Information on the labeling of quarternary packaging
Quaternary packaging is marked with a GS1-128 barcode containing the information GTIN (product identification), lot and expiry date. Next to, below or above the GS1-128 barcode, an HRI (Human Readable Interpretation or plain text line) is to be placed, which shows the encoded values. For quaternary packages, a one-dimensional symbol is used because it is assumed that such packages could be stored and retrieved by an automated logistics system that cannot necessarily process a two-dimensional symbol.
Barcodes for labeling medicinal products
For the labeling of medicinal products in Switzerland, in each case the three barcodes explained below can be used.
| Barcode | Data Content | Remarks |
| GTIN only | Can be read by 1D and 2D scanners Can be processed by all cash register systems with scanner in pharmacies. Application to secondary packaging |
|
|
(01)07612345002552 |
GTIN + attributes [GS1 Application Identifier Standard] |
Can only be read by 2D scanners Allows reading of information such as lot, EXP and serial number. Application to primary and secondary packaging |
| GTIN + attributes [GS1 Application Identifier Standard] |
Can be read by 1D and 2D scanners
|
What happens in the background when a barcode is read from a scanner?
Our explanatory film "Scanning a barcode" (Video in german) shows what action is triggered in an IT system after a barcode has been scanned. (Video in german)

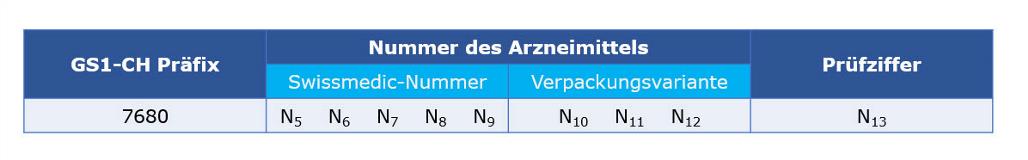
Identification of medicinal products with Swissmedic authorisation
The GTIN is assigned exclusively for products manufactured by Swissmedic approved drugs and immunobiological products with 5-digit registration numbers and 3-digit package codes.
This data element has the following structure:
Notice:
All other medicinal products authorized by Swissmedic must be identified by a company's owned GTIN.
The marketing authorization holder is free to use this national solution for the identification and labeling of medicinal products or to use their own GTINs. This solution was introduced in 1984 established to improve drug safety and processes in Switzerland.
Medicinal products products approved in Switzerland are included for basic referencing in the refdatabase: www.refdata.ch
More Information:
Effects due to the implementation of the FMD in Switzerland
On the implementation of the Falsified Medicine Directive (FMD) in Switzerland: As part of the revision of the Therapeutic Products Act (TPA), a number of measures defined. The specifications were made by the European Union and the Swiss Federal Council and aim to protect patients against counterfeit medicines in the legal supply chain. The legal basis for this is the Anti-Counterfeiting Directive 2011/62/EU, the Delegated Regulation (EU) 2016/161 as well as Art. 17a TPA and the related ordinance, Art. 17a TPA.
These new regulatory requirements only affect RX drugs, i.e. Medicine products assigned to Swissmedic Lists A and B.
Note: Effects due to Corona
Due to the Corona pandemic, the FOPH is currently unable to issue any binding statement when the ordinance on Article Art. 17a TPA will enter into force. The FOPH has received the comments on the relevant consultation published. Download.
Individual recognition features in the supply chain
identification features and safety devices on the packaging of medicines. More information on the topic.
A pseudo-randomized Serial number serves as a recognition feature on each secondary packaging, which is printied by means of a GS1 DataMatrix barcode and an HRI (Human Readable Interpretation).
Parallel to this, a verification system is being set up in which the manufacturers upload their serial number information before the products come into the supply chain.
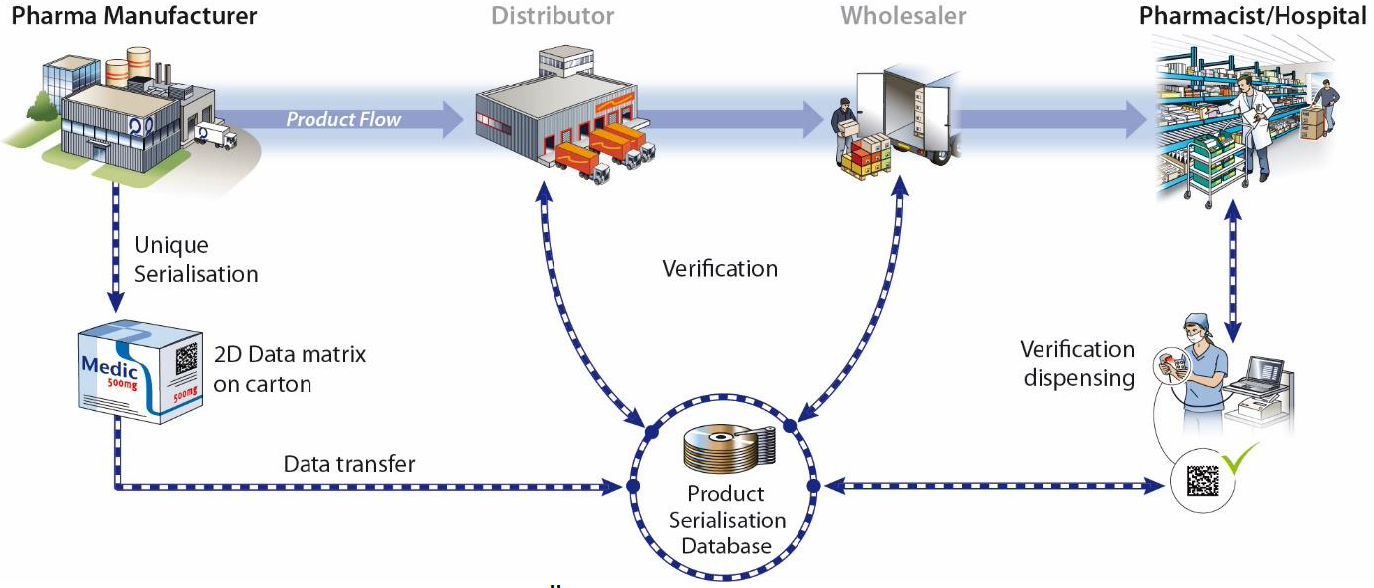
When dispensed at the pharmacy, hospital or doctor's office, the Data element on the package checked against the verification system and the respective package in the database as consumed marked. This system allows testing at all stations of the physical flow of goods and thus prevents the penetration of the neat supply chain with counterfeit products.
The safety devices on the packaging include in particular that in the future only packaging will be placed on the market be allowed to be "tamper proof" (first opening guarantee), i.e. a Packaging must be designed in such a way that it cannot be resealed.
The GS1 system allows medication and patient safety and simplifies the documentation in clinical information systems and increases the efficiency of the clinical patient pathway and the supply chain.
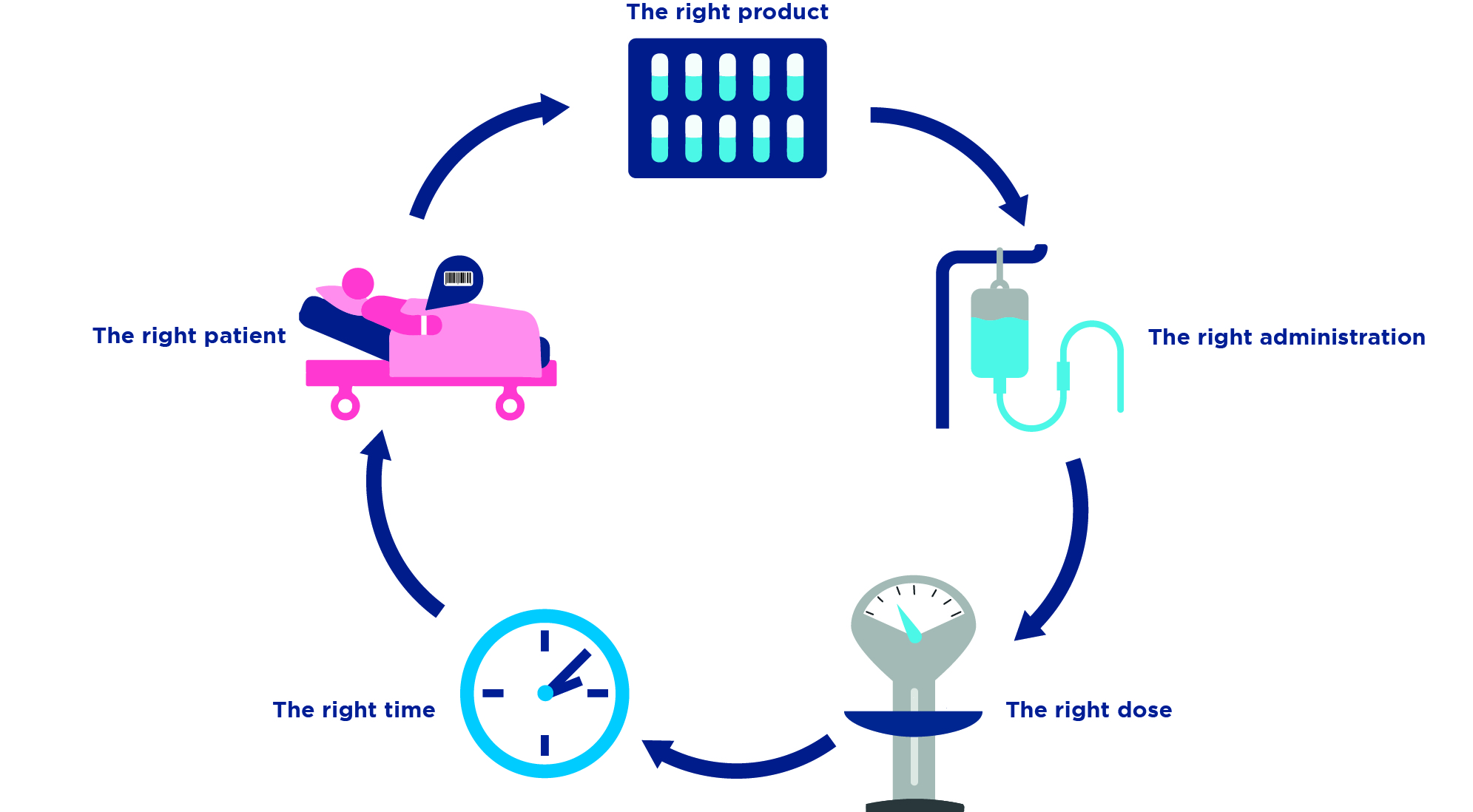
- The GS1 system is the basis for the digitization of the Health care.
- GS1 identification keys are used to identify pharmaceuticals, Patients, caregivers and locations clearly identified.
- With GS1 barcodes, identification and attributes are printed on every Object made available in machine and human readable form.
- With the master data exchange standard GDSN (Global Data Synchronization Network), master data can be shared across multiple parties and there is no need for time-consuming Mappings.
- The GS1 EDI standard simplifies the digitization of the Order-to-Cash Cycles.
Note
Do you need a pharmacode?
The Pharmacode can be obtained directly from HCI Solutions AG under the following coordinates: Koordinaten: www.hcisolutions.ch / Tel. 058 851 26 00