Project firstbase healthcare
In the focus sector healthcare, GS1 Switzerland defines a use case together with various representatives of the sector. The aim is to create a common basis for the future industry solution for the exchange of product master data. "HC Swiss DAP" ensures that the relevant information can be exchanged between all stakeholders in the Swiss healthcare sector without great effort.
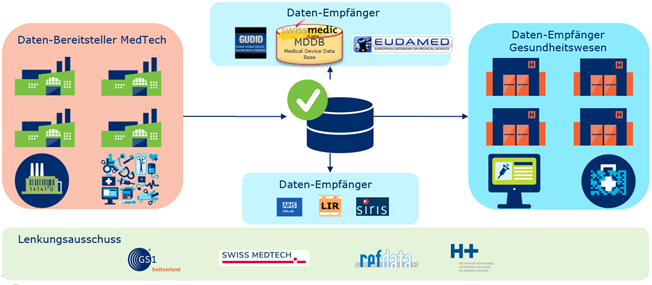
The new data exchange platform will offer all participants the possibility to exchange trustworthy product master data. Through various channels, the data senders will provide the data receivers with up-to-date, complete and correct master data "from the source".
With the experienced technology partner Bayard from Cologne, GS1 Switzerland has started the implementation and will realise the GS1 Switzerland data exchange platform based on the "byrd" platform in the coming months.
The use case "HC Swiss DAP" contributes to providing the Swiss healthcare system with up-to-date, correct and complete product data according to the "once only principle". To this end, a steering committee was initiated which, together with other representatives from the healthcare sector, ensures that the data exchange platform meets the needs of all stakeholders and is accepted in the market.
The steering committee, consisting of SWISS MEDTECH, H+, refdata and GS1 Switzerland, is involved in the following topics:
- Development of an industry solution for the electronic and automated exchange of master data of medical devices in the Swiss healthcare system.
- Communication and promotion of the solution in the market
- Beneficial solutions for the respective members.
The expert group Supply Chain Management Consumables, Medical Devices and Objects (SCM VMO) supports the project
The expert group SCM VMO, which focuses on the principles of digitisation - common semantics, common standards and interoperability - has supported the project from the beginning. Manufacturers of medical devices and hospitals from all over Switzerland are giving the go-ahead by actively participating in the "HC SWISS DAP" use case and thus implementing the project in the market. The insights gained will help to further optimise the platform functionalities and significantly shorten the implementation time for follow-on companies.
Supporting companies
The following hospitals support the project and make themselves available as data recipients for the first implementation phase with the goal of full integration:
On the industry side, the following companies support the planned data exchange platform:
Perspective
Firstbase healthcare is live. The first important milestone was reached successfully and on time. The "HC Swiss DAP" project can be transferred to operational use. In the coming months, all relevant stakeholders such as data suppliers (Swiss MedTech sector), data recipients (service providers/hospitals) and service providers will now be contacted. The goal is clear: firstbase healthcare is the industry solution for the exchange of relevant product master data in the Swiss healthcare sector. You too can support this industry solution and make a valuable contribution to the entire industry. You can find all further information on our website www.firstbase.ch.
Current status
January 2023
At the beginning of the year, the live environment of firstbase was finalised. On 23.1.2023, GS1 Switzerland was able to successfully go live with firstbase healthcare. Many hours of hard work were rewarded with the go-live. Thanks to the tireless efforts of the internal as well as external project team, this important milestone could be realised. Many thanks to all those involved for their valuable contributions!
Dezember 2022
Further test users will be brought onto the platform. The four hospitals Hirslanden, Inselspital, Kantonsspital Winterthur and Luzerner Kantonsspital define the first suppliers who can publish their product master data on the platform. Further accompanied onboarding webinars with the test users will follow.
The planned GS1 Switzerland platform will be published under the name firstbase. Information will be posted shortly at www.firstbase.ch.
November 2022
The test environment is put into operation. The use case organisations receive their logins and can explore the platform. In two online meetings, the test users get a first insight into the platform.
October 2022
In several coordination meetings, the data recipients and data senders have agreed on a common target market profile for the Swiss health sector. This will now be used as the basis for the planned data exchange platform and incorporated into the new solution. As soon as this work is completed, the first data senders will be able to make their data available to the data recipients via the data exchange platform. The organisations participating in the use case will be closely accompanied and supported by GS1 Switzerland and Bayard.
September 2022
Further organisations pledge their support. GS1 Switzerland is added to the list of data pools as a licensed GDSN data pool.
July 2022
In the "Medtech in Switzerland 2030" target vision, Swiss Medtech outlines a way in which Switzerland can permanently strengthen its attractiveness as a medtech location. In particular, the "Digitalisation" field of action in Swiss MedTech's strategy takes account of the project.
June 2022
The expert group SCM VMO has decided on joint implementation and is united behind the project. To consolidate this, a joint declaration of intent is signed.
The project was presented to a broad public for the first time at the Excellence Days on 8 and 9 June 2022. The presentation gave those present a comprehensive overview of the planned data exchange platform and its basic functions.
The solution provider Bayard, on whose technology the platform will be built, also presented itself and the solution approach. Peter Biedermann, Director of Swiss Medtech, once again emphasised the importance for the entire Swiss medical technology sector. The association sees digitisation as an opportunity very clearly.
The first hospitals and companies in the medical sector support the project and are making themselves available for the implementation phase.
The platform in brief
The basis for the mutual exchange of data according to the "once only principle" is a platform on which manufacturers make their product master data available directly from their systems. This product master data can be obtained by all connected companies (data recipients). The data is kept on the platform, verified and made available to the defined market participants. The data can also be shared with other databases.
UDI data
To register validated product information electronically, more is needed than its technical provision. Here, it is necessary to establish own processes, organisation and systems, which ensure the up-to-dateness and quality of the data in the long term. The Medical Device Regulation MDR obliges manufacturers of medical devices to store data about themselves and their products for Europe in EUDAMED. Medical devices offered in the USA must be registered electronically in the FDA's product database (GUDID) beforehand.
The future platform will ensure the registration of your products in the UDI databases. Be it the GUDID in the USA, the EUDAMED in Europe or one day the swissdamed (Swiss Database on Medical Devices) in Switzerland.
Implant register
In order to track implants quickly and efficiently, it is a legal requirement that products are registered in the Dutch Implant Register (LIR). This register can also be operated via the platform.
GS1 GDSN
GS1 GDSN (Global Data Synchronization Network) helps you to deliver your data to many of your national and international customers in a standardised and automated process. To improve the supply chain, the National Health Service (NHS), the largest purchaser of medical devices in the UK and Northern Ireland, requires device product data to be delivered electronically via GDSN. While this is not a regulatory requirement, it is essential for any device company marketing their products in the UK to understand and comply with. With the help of the platform, you can clear this hurdle too.
Do you want to learn more and stay informed about the progress of the use case? Then sign up to the newsletter here.